Abstract
Introduction Immune thrombocytopenia (ITP) is an autoimmune condition characterised by platelet destruction and insufficient platelet production. The American Society of Hematology ITP guidelines recommend rituximab, splenectomy, and thrombopoietin receptor agonists (TPO-RAs) as primary treatment options in patients with chronic ITP (Neunert C, et al. 2019). Fostamatinib, a spleen tyrosine kinase inhibitor, is approved for the treatment of chronic ITP in patients who have had an insufficient response to a previous treatment (FDA 2018, EMA 2020). Recent real-world evidence suggests that fostamatinib is used after a previous TPO-RA line (Hughes DM, et al. 2021). The objective of this network meta-analysis (NMA) was to estimate the relative efficacy of fostamatinib compared with current standard treatments after TPO-RAs in patients with refractory chronic ITP for overall platelet response.
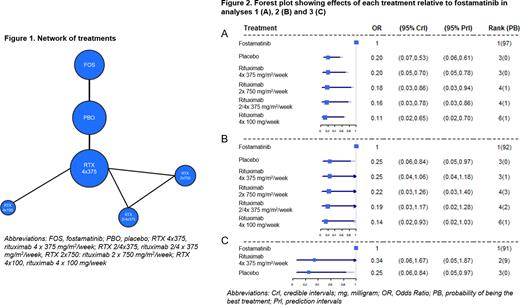
Methods A systematic literature review was conducted in 2019 and updated in July 2021. Six identified studies informed the NMA, including 2 placebo-controlled trials of fostamatinib (Bussel J, et al. 2018), 2 placebo-controlled trials of rituximab (Arnold DM, et al. 2012, Ghanima W, et al. 2015), 1 randomised trial of 3 rituximab regimens (Zwaginga JJ, et al. 2015) and 1 non-randomised study of 2 rituximab regimens (Zaja F, et al. 2012) (Figure 1). No studies assessing additional comparators of interest were found that could inform the NMA.
The outcome of interest was overall platelet response, however definitions varied across studies in terms of platelet count thresholds and assessment time points, and several analyses were therefore performed to investigate sensitivity in variation in outcome definition. Analysis 1 used the published definitions for each study. Analysis 2 used an alternative standardised definition (platelet count >30,000/µL by week 4) for the fostamatinib trials, and the published definitions for each of the remaining studies. Analysis 3 used the alternative standardised definition for all studies and was restricted to those with results available: Bussel 2018 and Ghanima 2015.
Possible treatment effect modifiers were evaluated by 8 British haematologists via a Delphi panel. None were identified and it was assumed that the differing baseline characteristics between studies did not have a significant impact on the outcome of interest.
Random-effect models were employed in the analyses. The Odds Ratio (OR) for outcome occurrence of each treatment compared to fostamatinib together with the 95% credible intervals (CrI), 95% prediction intervals (PrI), median rank and probability of being the best treatment were estimated and are displayed in forest plots for each analysis. ORs<1 indicate reduced response relative to fostamatinib. All analyses were performed following the guidance of the National Institute for Health and Care Excellence Decision Support Unit and were implemented with WinBUGS and R.
Results In analysis 1, pairwise comparisons indicated that fostamatinib was statistically significantly more effective than placebo and all rituximab regimens with ORs for comparators vs. fostamatinib ranging from 0.11 to 0.20 (Figure 2A).
In analysis 2, fostamatinib was associated with improved response vs. placebo and all rituximab regimens (OR range 0.14 - 0.25) but statistical significance was only reached vs. placebo and rituximab 4 x 100 mg/week (Figure 2B).
In analysis 3, pairwise comparisons suggested increased efficacy of fostamatinib relative to placebo and rituximab 4 x 375 mg/m2/week with ORs of 0.25 and 0.34, respectively. However, OR was only statistically significant vs. placebo (Figure 2C).
The probability of fostamatinib being the most effective treatment ranged 0.91 - 0.97 across analyses; median rank for fostamatinib was 1 in all analyses (Figure 2).
The between study standard deviations indicated moderate heterogeneity in treatment effects between studies.
Conclusions The results from all analyses indicate that fostamatinib was associated with improved overall platelet response relative to rituximab at all regimens evaluated (4 x 375 mg/m2/week, 2/4 x 375 mg/m2/week, 2 x 750 mg/m2/week, 4 x 100 mg/week), and placebo. The use of rituximab offered no additional benefit with regards to achieving a platelet response than being on placebo. Fostamatinib may be a better alternative than rituximab to increase platelet counts in patients with refractory chronic ITP.
Disclosures
Laws:Grifols: Consultancy. Gomez-Ulloa:Grifols: Current Employment; IQVIA: Ended employment in the past 24 months. Calvo:Grifols: Current Employment. Stacey:Grifols: Current Employment. McDonald:Grifols: Research Funding; Abbvie: Consultancy, Speakers Bureau; Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel to conferences, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; argenx: Other: travel to conferences; Rigel: Research Funding.
OffLabel Disclosure:
Rituximab use in chronic immune thrombocytopenia
Author notes
Asterisk with author names denotes non-ASH members.